Nanopore-confined Bilayers: a Model of Biomembranes with Defined Curvature and a Tool for Oriented Sample Magnetic Resonance
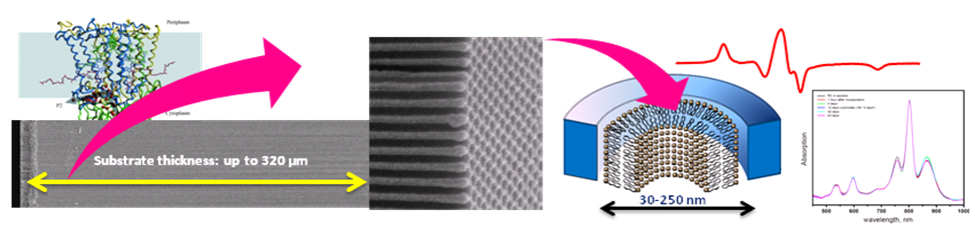
Figure 1. A cartoon of lipid nanotube arrays and incorporation of functional RC in nanotubular bilayers. Left Panel: (Top) – a ribbon diagram of a photosynthetic reaction center (RC) protein from purple bacterium Rhodobacter Sphaeroides; (Bottom) – a scanning electron microscopy (SEM) image of the entire 320 μm cross-section of the AAO substrate fabricated at NCSU. Center Panel: a close-up SEM of the AAO surface shows highly ordered hexagonally packed pores with an average diameter of 48 nm. Right Panel: a cartoon of a lipid nanotube formed inside a nanopore and an EPR spectrum of spin-labeled peptide gramicidin A (top, red) showing a high degree of macroscopic alignment; stability of membrane proteins in such matrices could be accessed by conventional UV-vis spectroscopy as demonstrated for RC protein that remained at least 90% stable over the period of 63 days (bottom right).It is well established that biological function of cellular membranes is determined by both membrane proteins and the membrane biochemical composition. Moreover, the membrane shape/local curvature may also play a role in modulating membrane properties and the protein function.
While specific biomembrane compositions are more readily replicated in biophysical bench experiments, formation of bilayers of specific shapes and curvature represents a more challenging task. Macroscopic alignment of such membranes – an important prerequisite of high resolution magnetic resonance and other studies – is even more difficult especially over a broad range of experimental conditions such pH, ionic strength, and temperature.
Our lab developed methods for forming self-assembled lipid nanotubular bilayers inside cylindrical nanopores composed of anodic aluminum oxide (AAO). Such hybrid nanostructures, named lipid nanotube arrays, represent a new type of substrate-supported and macroscopically-aligned lipid bilayers of defined curvature that have many attractive features for both membrane biophysics and structure-function protein studies by spectroscopic techniques. Optical properties of AAO allow for assessing the integrity of membrane protein complexes by UV-vis while high density of the deposited lipids and proteins enable examination by other biophysical methods, including DSC, QCM, and magnetic resonance. The latter studies have shown that the individual lipids in such nanopore-confined structures maintain fast uniaxial diffusion and a high degree of macroscopic alignment. The macroscopic alignment enables detailed studies of effects of lipid composition on structure of integral membrane proteins by solid state oriented sample NMR, EPR and DEER. Accessibility of either both or mainly inner leaflet of the nanotubular bilayers to water-soluble species provides for studies of protein, peptides, and drug binding.
